Research Article
Open Access
Fine Structure of the Gas Bladder of Alligator
Gar, Atractosteus spatula
Ahmad Omar-Ali1, Wes Baumgartner2, Peter J. Allen3, Lora Petrie-Hanson1*
1Department of Basic Sciences, College of Veterinary Medicine, Mississippi State University, Mississippi State, MS 39762, USA
2Department of Pathobiology and Population Medicine, College of Veterinary Medicine, Mississippi State University, Mississippi State, MS 39762, USA
3Department of Wildlife, Fisheries and Aquaculture, College of Forest Resources, Mississippi State University, Mississippi State, MS 39762, USA
2Department of Pathobiology and Population Medicine, College of Veterinary Medicine, Mississippi State University, Mississippi State, MS 39762, USA
3Department of Wildlife, Fisheries and Aquaculture, College of Forest Resources, Mississippi State University, Mississippi State, MS 39762, USA
*Corresponding author: Lora Petrie-Hanson, Associate Professor, College of Veterinary Medicine, 240 Wise Center Drive PO Box 6100, Mississippi
State, MS 39762, USA, Tel: +1-(601)-325-1291; Fax: +1-(662)325-1031; E-mail:
@
Received: 1 November, 2016;Accepted: 2 December, 2016 ; Published: 12 December, 2016
Citation: Omar-Ali A, Baumgartner W, Allen PJ, Petrie-Hanson L (2016) Fine Structure of the Gas Bladder of Alligator Gar, Atractosteus
Spatula. Int J Sci Res Environ Sci Toxicol 1(1): 8.
Abstract Top
Anthropogenic factors seriously affect water quality and
adversely affect fish populations. Agricultural run-off accumulates
in the Mississippi River and the coastal estuaries. Alligator gar
(Atractosteus spatula) inhabits these waters and is impacted by
agricultural pollution, petrochemical contaminants and oil spills.
These fish are bimodal air breathers, and use a primitive lung as an
accessory organ. The gas bladder, or Air Breathing Organ (ABO) of
alligator gar (Atractosteus spatula), is a vascularized air sac located
dorsal to the body lumen. It has characteristics of amphibian lungs.
Alligator gar air-breathing organs function to supplement branchial
respiratory exchange with aerial gas exchange. The alligator gar gas
bladder is an elongated air sac that originates dorsal to the pharynx.
Air enters through a pharyngeal-esophageal duct that is controlled by
two glottal ridges. The pharyngeo-esophageal duct is approximately
5mm long and is lined by ciliated columnar epithelium that is
continuous with the central canal epithelium. The gas bladder has
a central canal that subdivides the bladder into right and left lobes.
Each lobe is further divided by septa into series of air spaces. The
septa consist of blood vessels and smooth and striated muscles.
Air circulates throughout the central canal, lobes and air spaces.
The thickness of the septa is determined by underlying, supportive
striated muscle. The internal surface of the gas bladder is a continuous
respiratory epithelial layer that includes mucus cells, pneumocytes,
and ciliated epithelial cells. An understanding of the normal tissue
characteristics of this air breathing organ provides a baseline for
studying the effects of environmental toxins on this organ.
Keywords: Alligator gar; Atractosteus spatula; Air breathing organ, Gas bladder; Pharyngeo-esophageal duct
Keywords: Alligator gar; Atractosteus spatula; Air breathing organ, Gas bladder; Pharyngeo-esophageal duct
Introduction
Alligator gar, Atractosteus spatula, is member of the
Infraclass Holostei. This group includes primitive fish and
is phylogenetically placed between the chondrosteans and
the teleosts [1]. Holostei arose around 180 million years ago
[2,3], and includes the orders Amiiformes, the bowfin, and
Lepisosteiformes, the gars. These fish have not been well studied
[4]. Gars occur in North and Central America, as well as in Cuba.
The alligator gar is the largest of the gars (nearly 3 meters
maximally) [5]. Within the order Lepisosteiformes, the family Lepisosteidae includes the genera Atractosteus and Lepisosteus.
Atractosteus includes A. spatula (alligator gar), A. tristoechus
(Cuban gar), and A. tropicus (tropical gar), while Lepisosteus
includes L. oculatus (spotted gar), L. osseus (long nose gar), L.
platostomus (short nose gar), and L. platyrhincus (Florida gar)
[2,6,7]. Atractosteus are distinguishable from Lepisosteus by
shorter, more numerous gill rakers and a more prominent
second row of teeth in the upper jaw [2]. Atractosteus spatula
are distributed in lakes, rivers and estuaries along the coast of
the northern Gulf of Mexico [3,8,9]. Currently, estimated gar
numbers and fishing regulations vary by state. In Louisiana,
alligator gar numbers have decreased [5].
Air breathing fishes occur in fresh and salt water [1] and their aquatic habitats range from desert to tropical rain forest. Amphibians evolved to breathe air while inhabiting aquatic or terrestrial environments. In contrast, bimodal air breathing fish evolved to breathe by exchanging gases from the water and the atmosphere [1], while inhabiting water. Obligatory air breathing fishes use only aerial gas exchange and bimodal air breathing fishes use primarily aerial gas exchange [10-13]. Bimodal and obligatory air breathers can utilize branchial and Air Breathing Organ (ABO) respiration simultaneously [1]. Gars are bimodal air breathing fish that use their gas bladder as a respiratory organ to extract oxygen from the atmosphere [1], especially in hypoxic environments [14]. Fish gills have a large surface area and are in direct contact with the environment. They have important functions including respiration, ion regulation, excretion of nitrogenous waste, and gas exchange [15-18]. Gill surface areas and lamellar thickness are reduced in air breathing fishes in comparison to non-air breathing fishes, but still function for both O2 uptake and most of CO2 excretion [19-22].
The gas bladder of air breathing fish has histological features of amphibian lungs [23,24]. Although the fish ABO and amphibian lungs function similarly, these two organs develop ontogenetically from different tissues [25]. Embryologically, the gas bladder originates from the pharyngeo-esophageal area as a dorsal diverticulum of the esophagus and is joined to the esophagus by the pneumatic duct [26,27]. This duct remains in fish with a physostomous air bladder, but is absent in adult fish with a physoclistic air bladder [28]. There is controversy surrounding the presence or absence of a pneumatic duct in lepisosteid fish (reviewed in [29]. The bladder itself contains numerous septa that provide a large vascularized area for gas exchange [30]. There is also abundant smooth and skeletal muscle throughout the gas bladder. It is attached to the body wall by dorsal retractors, allowing for contraction.
Most studies on the physiology of gars have utilized Lepisosteus species [1,30]. Such studies confirm the function of the bladder in oxygen uptake and carbon dioxide release and its necessity when fish are in hypoxic waters [1,14,22]. Similar functions and presumably physiology are seen in Atractosteus spatula [1,31].
Alligator gar is exposed to petrochemical pollutants and agricultural runoff because of the environments they inhabit. Furthermore, as top predators they can bio accumulate pollutants. Alligator gar were evaluated in our studies following the Gulf of Mexico oil spill [32,33].To our knowledge, the fine structure of the alligator gar air breathing organ has not been documented. The purpose of this study was to histologically describe the alligator gar ABO using light and electron microscopy. Knowledge of this organ is necessary to evaluate the effects of pollutants on alligator gar. The ABO is the focus of our on-going studies of this unusual fish.
Air breathing fishes occur in fresh and salt water [1] and their aquatic habitats range from desert to tropical rain forest. Amphibians evolved to breathe air while inhabiting aquatic or terrestrial environments. In contrast, bimodal air breathing fish evolved to breathe by exchanging gases from the water and the atmosphere [1], while inhabiting water. Obligatory air breathing fishes use only aerial gas exchange and bimodal air breathing fishes use primarily aerial gas exchange [10-13]. Bimodal and obligatory air breathers can utilize branchial and Air Breathing Organ (ABO) respiration simultaneously [1]. Gars are bimodal air breathing fish that use their gas bladder as a respiratory organ to extract oxygen from the atmosphere [1], especially in hypoxic environments [14]. Fish gills have a large surface area and are in direct contact with the environment. They have important functions including respiration, ion regulation, excretion of nitrogenous waste, and gas exchange [15-18]. Gill surface areas and lamellar thickness are reduced in air breathing fishes in comparison to non-air breathing fishes, but still function for both O2 uptake and most of CO2 excretion [19-22].
The gas bladder of air breathing fish has histological features of amphibian lungs [23,24]. Although the fish ABO and amphibian lungs function similarly, these two organs develop ontogenetically from different tissues [25]. Embryologically, the gas bladder originates from the pharyngeo-esophageal area as a dorsal diverticulum of the esophagus and is joined to the esophagus by the pneumatic duct [26,27]. This duct remains in fish with a physostomous air bladder, but is absent in adult fish with a physoclistic air bladder [28]. There is controversy surrounding the presence or absence of a pneumatic duct in lepisosteid fish (reviewed in [29]. The bladder itself contains numerous septa that provide a large vascularized area for gas exchange [30]. There is also abundant smooth and skeletal muscle throughout the gas bladder. It is attached to the body wall by dorsal retractors, allowing for contraction.
Most studies on the physiology of gars have utilized Lepisosteus species [1,30]. Such studies confirm the function of the bladder in oxygen uptake and carbon dioxide release and its necessity when fish are in hypoxic waters [1,14,22]. Similar functions and presumably physiology are seen in Atractosteus spatula [1,31].
Alligator gar is exposed to petrochemical pollutants and agricultural runoff because of the environments they inhabit. Furthermore, as top predators they can bio accumulate pollutants. Alligator gar were evaluated in our studies following the Gulf of Mexico oil spill [32,33].To our knowledge, the fine structure of the alligator gar air breathing organ has not been documented. The purpose of this study was to histologically describe the alligator gar ABO using light and electron microscopy. Knowledge of this organ is necessary to evaluate the effects of pollutants on alligator gar. The ABO is the focus of our on-going studies of this unusual fish.
Materials and Methods
Animals
Nine alligator gar were obtained from the Private John Allen
National Fish Hatchery in Tupelo, MS, and held in fresh water
at the Mississippi Agriculture and Forestry Experiment Station,
South Farm Aquaculture Unit, following published methods
[34].When sampled, the fish weighed 318 to 320 gm and
measured 425 to 430 mm in length. For tissue collection, each
fish was placed in an overdose of anesthetic (500 mg/L tricaine
methanesulfonate). The gills, body wall and other viscera were
removed for analysis of the pharyngeo-esophageal junction.
The MSU Institutional Animal Care and Use Committee (IACUC)
approved fish holding and experimental protocols.
Light microscopy
Samples were rinsed in physiological saline and fixed in
phosphate buffered 10% formalin. Tissues were processed
and embedded in paraffin, sectioned at 4 μm, and stained
with hematoxylin and eosin (H&E) and Alcian blue stain.
Immunohistochemistry for smooth muscle actin was performed
on sections using a Dako Autostainer using a biotinylated
streptavidin antibody detection system (Dako LSAB2) with DAB
chromogen and hematoxylin counterstain. A monoclonal mouse,
anti-human smooth muscle actin primary antibody was used
(Dako Clone 1A4, code M0851, 1:100 dilution) and for a negative
control, mouse IgG1 was used. Deparaffinization was followed
by hydration (1x Tris pH 6.0) for 5 minutes, then steamed for
30 minutes (antigen retrieval), with subsequent automated
processing with primary antibody, and DAB chromogen. Slides were then rinsed in deionized water, dipped in 0.3% ammonia
hydroxide, rinsed again in deionized water, dehydrated through
graded alcohol, stained with hematoxylin and eosin, and cover
slipped. Slides were viewed under light microscopy on an
Olympus BX 51 (Olympus America Inc.) and photographed using
Picture Frame™ software.
Transmission Electron Microscopy (TEM)
Samples were primarily fixed in 2% glutaraldehyde in
cacodylate buffer (0.1 M phosphate buffer, pH 7.2) and then post
fixed in 2% osmium tetroxide at 4°C. Samples were dehydrated
in solutions of increasing ethanol concentration from 35%
to 100% ETOH, and then in solutions of increasing acetone
concentration. Finally, samples were embedded in Spurr’s resin.
Ultra-sections were made from each block using a Reichert Jung
ultra-microtome and stained with toluidine blue stain for thick
sections or uranyl acetate and lead citrate for the ultra-thin
sections [35,36]. All sections were viewed under transmission
electron microscopy on a JEOL JEM-1230 at 80 kV.
Results
Gross Morphology
The alligator gar gas bladder is a large, dorsoventrally
flattened, elongate organ that is intimately associated with the
musculoskeletal tissues of the dorsal coelom along its entire
length and width (Figure A, G). The bladder originates at the
abrupt termination of the pharyngeo-esophageal duct (Figure
1D, asterisks) and is attached to the body wall by paired slender
collagenous folds that run along its entire length on either side
of the vertebral column, blending into the stroma that invests
the aorta and cardinal veins associated with the mesonephros
(Figure 1G). The width of the gas bladder is proportional to the
width of the body, tapering and terminating at the end of the
coelom (Figure A, B).
The alligator gar gas bladder is a large, dorsoventrally flattened, elongate organ that is intimately associated with the musculoskeletal tissues of the dorsal coelom along its entire length and width (Figure A, G). The bladder originates at the abrupt termination of the pharyngeo-esophageal duct (Figure 1D, asterisks) and is attached to the body wall by paired slender collagenous folds that run along its entire length on either side of the vertebral column, blending into the stroma that invests the aorta and cardinal veins associated with the mesonephros (Figure 1G). The width of the gas bladder is proportional to the width of the body, tapering and terminating at the end of the coelom (Figure 1 A, B). Grossly, the bladder’s rich vascular supply can be easily appreciated with finely arborescent vessels throughout, except for a narrow midline strip of poorly vascularized tissue running along its ventral length (Figure 1A, B). This strip delineates a continuous median central canal that divides the organ into symmetrical halves (Figure 1A, B, and F). The roof and the floor of this central canal are delicate, flat, fibrous membranes. The roof blends into the fibrous raphe that attaches the organ to the aorta (Figure 1G).
Each half of the gas bladder is subdivided by smoothly interconnected, progressively finer and shorter septa forming
The alligator gar gas bladder is a large, dorsoventrally flattened, elongate organ that is intimately associated with the musculoskeletal tissues of the dorsal coelom along its entire length and width (Figure A, G). The bladder originates at the abrupt termination of the pharyngeo-esophageal duct (Figure 1D, asterisks) and is attached to the body wall by paired slender collagenous folds that run along its entire length on either side of the vertebral column, blending into the stroma that invests the aorta and cardinal veins associated with the mesonephros (Figure 1G). The width of the gas bladder is proportional to the width of the body, tapering and terminating at the end of the coelom (Figure 1 A, B). Grossly, the bladder’s rich vascular supply can be easily appreciated with finely arborescent vessels throughout, except for a narrow midline strip of poorly vascularized tissue running along its ventral length (Figure 1A, B). This strip delineates a continuous median central canal that divides the organ into symmetrical halves (Figure 1A, B, and F). The roof and the floor of this central canal are delicate, flat, fibrous membranes. The roof blends into the fibrous raphe that attaches the organ to the aorta (Figure 1G).
Each half of the gas bladder is subdivided by smoothly interconnected, progressively finer and shorter septa forming
Figure 1:Atractosteus spatula. A: Ventro-dorsal view of the air-breathing
organ (ABO), with gills, body wall, and other viscera removed. The
head is to the right. Arrow indicates the central lumen, asterisks indicate
the air exchange fields. Formalin fixed specimen. B: Ventro-dorsal
view of the ABO, fresh specimen, and head is to the right. Arrow and
asterisks denote features similar to that in A. C: Ventro-dorsal view of
the ABO, with the ventral half of the organ removed. Head is to the top
of the page. Arrow denotes the central canal, dorsal aspect. Asterisks
denote primary and secondary septa. Formalin fixed specimen. D: Caudo-
rostral view of the ABO, transversely sectioned approximately 2cm
from the end of the pharyngeo-esophageal duct. Asterisks denote the
termination of the glottal ridges, arrow indicates the gas exchange surface.
Dorsum is at the top of the page. Formalin fixed specimen. E: Dorso-
ventral view of the ventral half of the ABO, with the dorsum removed
(opposite portion of the organ compared to C). Arrow denotes the floor
of the central canal. Asterisks denote primary septa. Formalin fixed
specimen. F: Caudo-rostral view of the ABO, transversely sectioned; left
lobe (arrow), septa (asterisks), the central canal is completely opened
in comparison to figure D. Formalin fixed specimen. G: Photomicrograph
of a transverse section of the entire ABO, dorsum towards the
top. Hematoxylin and eosin.
Figure 2:Atractosteus spatula, pharyngeo-esophageal duct. A: Gross
view of the dorsum of the pharyngeo-esophageal junction, head is to the
right, esophagus transected at the left, the ventral portion of the organ
removed. Blue line indicates the level of sectioning shown in B. Purple
line indicates the level of sectioning shown in C. Green line indicates the
level of sectioning shown in D. Formalin fixed specimen. B: Photomicrograph,
transverse section of pharyngeo-esophageal slit denoted by blue
line in figure A. A slit-like opening lined by a thick transitional epithelium
(black arrow and inset) connects the gut lumen (bottom) to the beginning
of the duct (top, green arrow). The glottal ridges are denoted by
the green, blue, and yellow arrows, which point to the epithelium, connective
tissue stroma, and muscle bundles, respectively. Hematoxylin
and eosin, 20 x. C: Photomicrograph, transverse section of pharyngeoesophageal
duct denoted by purple line in figure A. The inner aspect is
lined by a tall simple to pseudostratified columnar epithelium (black
arrow and inset). The lumen is surrounded on all sides by thick myocollagenous
stroma. Hematoxylin and eosin, 20 x. D: Sub gross photomicrograph,
transverse section of pharyngeo-esophageal duct denoted by
green line in figure A, at the duct termination. The small black arrow
indicates the termination of the glottal ridges, as seen in figure 1 D, asterisks.
A ring of skeletal muscle and tracts of smooth muscle delimit
the duct. The large black arrow denotes the esophagus, the green arrow
denotes the air bladder proper, hematoxylin and eosin.
first, second, third, and fourth order alveolar chambers (Figure
1C, E, G). Septa tend to have abrupt bulbous terminations
composed of dense stroma (Figure 3B). The central canal is
laterally delimited by the primary septa, forming an arcade of
regular pillars with wide intervening ostia that lead into the
gas exchange spaces. The progressing septal order divisions
often form oblique angles, with a reduction in septal height
by approximately half, finally terminating in low alveoli
approximately 1 millimeter wide (Figure 1C, E).
Microscopic Morphology and Ultra structure
In the glottis and pharyngeo-esophageal duct, the
pharyngeal mucosa is stratified columnar epithelium that varies
in thickness from 100 to 200 micrometers and predominantly
contains large mucus cells (Figure 2B, C). The glottal ridges are
the protruding lips of mucosa that guard the entrance to the
pharyngeo-esophageal duct. Anteriorly on transverse section,
the duct forms a “T” where a 2 to 3 millimeter long stem dorsally
meets a collapsed tubule forming the bar of the “T” (Figure 2B).
Progressing caudally as the slit opening ends (Figure 2C), the
duct continues to meet the gas bladder proper.Anteriorly, pharyngeal mucosa lines the duct. Initially,
a ciliated stratified columnar epithelium (esophageal type)
appears in the stem of the duct, becoming the predominant cell
type caudally as the duct becomes detached from the ventral
esophagus and eventually entirely lining the duct (Figure 2B, C,
D).
In the alligator gar pharyngeo-esophageal duct, a thick cushion of mucinous areolar connective tissue containing evenly spread, fine collagen fibrils with plump stellate mesenchymal cells supports the duct mucosa’s (Figure 2B and C). Subjacently, this stroma blends into dense fascicles of skeletal muscle admixed with small nerves and vessels. A thin layer of transverse and obliquely arranged skeletal muscle bundles overlie the dorsum of the duct where it abuts the vertebral column, along with symmetrical, anteroposteriorly arranged thick muscle bundles (dorsal retractors).
The floor of the central canal and ventral aspects of the primary septa are lined by a stratified to pseudo stratified, ciliated columnar epithelium with a prominent mucus cell component. The mucus cells contain PAS (not shown) and Alcian blue positive content, consistent with acid mucopolysaccharide (Figure 3I).
Progressing from first to fourth order septa, the epithelium gradually thins, with eventual loss of ciliated cells, giving way to a simple squamous epithelium covering capillaries and relatively few, evenly spaced, attenuated mucus cells (Figure 3C, D, E). This progression continues with eventual loss of mucus cells in the respiratory exchange surface (respiratory epithelium) (Figure 3E, F, G) (Figure 5A). This progressive epithelial change also occurs centrally to the periphery, where respiratory epithelium covers all alveolar surfaces; typical pneumocytes occur here. The respiratory surface is covered by irregular microvilli on squamous cells (Figure 4A, B, C). Electron micrographs demonstrate that squamous cells contain numerous lamellar bodies with stacked content (Figure 4C). Intercellular connections are composed of tight junctions and desmosomes with marked invagination of both adjacent cell membranes. Nuclei are irregular with a distinct thin rim of heterochromatin, abundant euchromatin, and prominent nucleoli (Figure 4A, B, C) (Figure 5A).
Mucus cell content was hyper chromatic (Figure4 A, B, C). Mucus cells lining alveoli and septal walls included occasional Neuroendocrine cells (NE) either between cells or along the basal aspects (Figure 4A). Neuroendocrine cytoplasm contained many dense uniform granules approximately 100 nm wide. Occasionally, NE cells were present in small tightly packed groups associated with nerves (Neuroepithelial Bodies-NEBs).
The central canal, septa, and alveoli consist of a similar sub epithelial stroma, composed of a thin layer of densely packed collagen fibers with fibroblasts and small blood vessels. The bulk of the septa, in particular the bulbous terminae, are composed of densely packed, irregularly arranged fascicles of striated skeletal muscle (Figure 3I). Smooth muscle is also prominent, investing septal skeletal muscle bundles with particular prominence
In the alligator gar pharyngeo-esophageal duct, a thick cushion of mucinous areolar connective tissue containing evenly spread, fine collagen fibrils with plump stellate mesenchymal cells supports the duct mucosa’s (Figure 2B and C). Subjacently, this stroma blends into dense fascicles of skeletal muscle admixed with small nerves and vessels. A thin layer of transverse and obliquely arranged skeletal muscle bundles overlie the dorsum of the duct where it abuts the vertebral column, along with symmetrical, anteroposteriorly arranged thick muscle bundles (dorsal retractors).
The floor of the central canal and ventral aspects of the primary septa are lined by a stratified to pseudo stratified, ciliated columnar epithelium with a prominent mucus cell component. The mucus cells contain PAS (not shown) and Alcian blue positive content, consistent with acid mucopolysaccharide (Figure 3I).
Progressing from first to fourth order septa, the epithelium gradually thins, with eventual loss of ciliated cells, giving way to a simple squamous epithelium covering capillaries and relatively few, evenly spaced, attenuated mucus cells (Figure 3C, D, E). This progression continues with eventual loss of mucus cells in the respiratory exchange surface (respiratory epithelium) (Figure 3E, F, G) (Figure 5A). This progressive epithelial change also occurs centrally to the periphery, where respiratory epithelium covers all alveolar surfaces; typical pneumocytes occur here. The respiratory surface is covered by irregular microvilli on squamous cells (Figure 4A, B, C). Electron micrographs demonstrate that squamous cells contain numerous lamellar bodies with stacked content (Figure 4C). Intercellular connections are composed of tight junctions and desmosomes with marked invagination of both adjacent cell membranes. Nuclei are irregular with a distinct thin rim of heterochromatin, abundant euchromatin, and prominent nucleoli (Figure 4A, B, C) (Figure 5A).
Mucus cell content was hyper chromatic (Figure4 A, B, C). Mucus cells lining alveoli and septal walls included occasional Neuroendocrine cells (NE) either between cells or along the basal aspects (Figure 4A). Neuroendocrine cytoplasm contained many dense uniform granules approximately 100 nm wide. Occasionally, NE cells were present in small tightly packed groups associated with nerves (Neuroepithelial Bodies-NEBs).
The central canal, septa, and alveoli consist of a similar sub epithelial stroma, composed of a thin layer of densely packed collagen fibers with fibroblasts and small blood vessels. The bulk of the septa, in particular the bulbous terminae, are composed of densely packed, irregularly arranged fascicles of striated skeletal muscle (Figure 3I). Smooth muscle is also prominent, investing septal skeletal muscle bundles with particular prominence
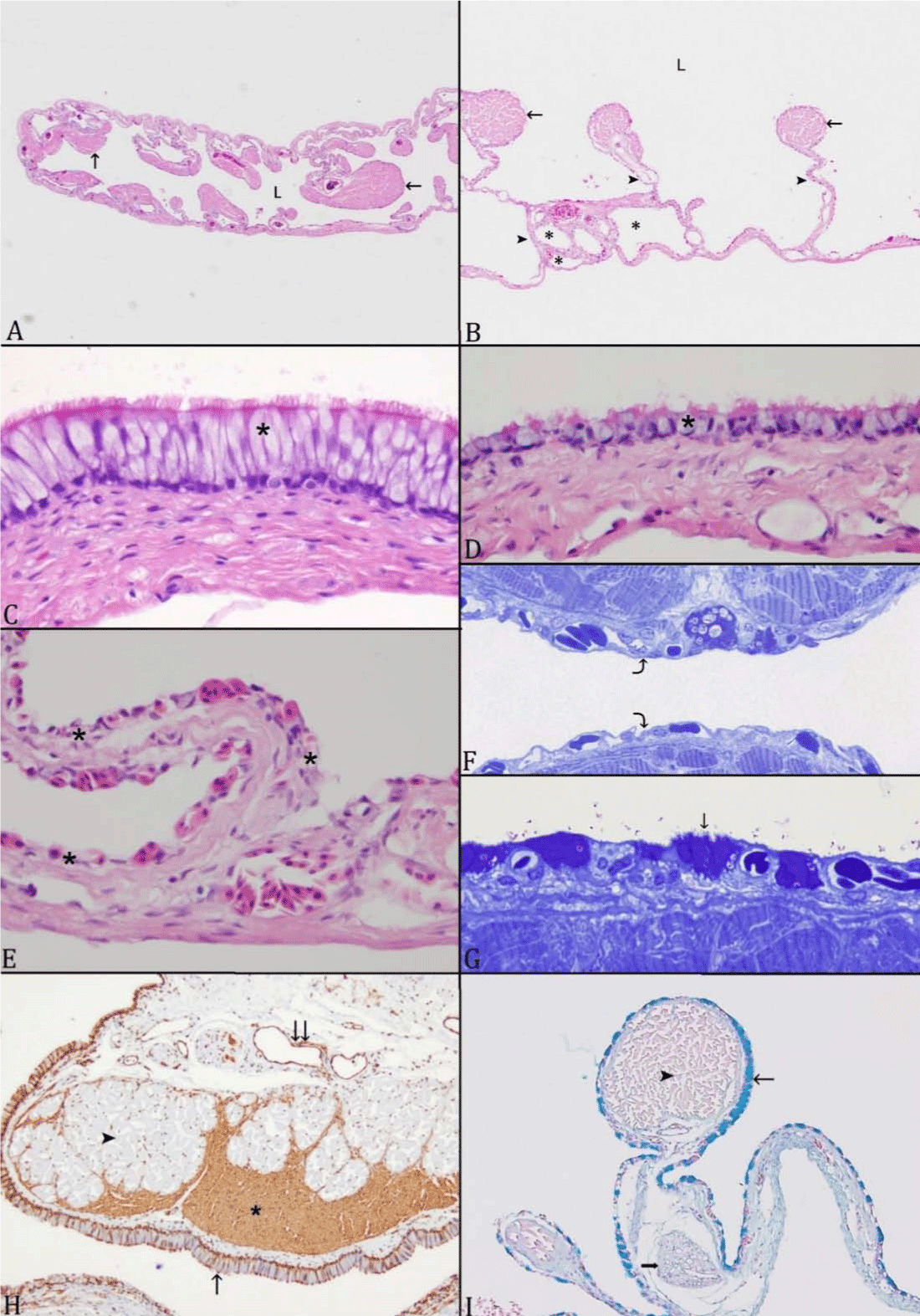
Figure 3:Multiple views of Atractosteus spatula. A: An overview of the
left lobe shows the lumen and septa. Septa are composed of striated
muscles, blood vessels, and forms bundles (arrow) that are projecting
toward the lumen; lumen (L). B: Each lobe is subdivided into different
levels of air spaces (asterisk) by series of septa (arrowhead); bundles of
striated muscle are in the apical portion of septa (arrow). C and D: The
internal surface is lined by a stratified to pseudostratified, ciliated columnar
epithelium with prominent mucus cell components (asterisk).
E: The internal surface gradually changes to simple squamous epithelium
rich with blood vessels (asterisk) (curved arrow).F: resin toluidine
blue stain section showing the same G:Simple squamous epithelium
rich with blood vessels with few, evenly spaced, attenuated mucus cells
(arrow); resin toluidine blue stain section. H: Cross section stained with
smooth muscle actin stain; smooth muscle (asterisk) is highlighted by
the antigen, and interdigitates with striated muscle (arrowhead); veins
are also lined by smooth muscle (double arrow). The internal surface is
lined by a stratified to pseudostratified, ciliated columnar epithelium
with prominent mucus cells components (arrow). I: Mucus cells contain
Alcian blue positive content (thin arrow), striated muscles (arrowhead);
nerve fiber (thick arrow); (Alcian Blue stain).
along their proximal aspects (Figure 3H). Unlike skeletal muscle,
smooth muscle is present throughout the stroma at all levels
and in general, forms delicate fascicles and individualized cells
that admix with connective tissues. Muscle content gradually
decreases caudally and peripherally.
Vessels, nerves, and unmyelinated axons run along the apical portions of the septal terminae, typically subjacent to the muscle bundles (Figure. 3A, B, F, G, I) (Figure 4A, D) (Figure 5C). Capillaries are regularly nestled between epithelial cells and are covered apically by thin extensions of epithelial cells, forming an air-blood barrier approximately 1 micron thick (Figure 4A, B). The endothelium is continuous, overlying a collagenous lamina. Endothelial nuclei are located away from the surface and have irregular nuclei with rims of heterochromatin (Figure. 4A, B). Within the delicate stroma of the floor of the central cavity, regular paired ganglia travel along the length of the organ bilaterally. Progressively smaller nerves without neurons are present in the septa as they become finer. There are few small, random aggregates of lymphocytes that surround blood vessels and encroach on the epithelium with occasional areas of epithelial intercellular migration.
Vessels, nerves, and unmyelinated axons run along the apical portions of the septal terminae, typically subjacent to the muscle bundles (Figure. 3A, B, F, G, I) (Figure 4A, D) (Figure 5C). Capillaries are regularly nestled between epithelial cells and are covered apically by thin extensions of epithelial cells, forming an air-blood barrier approximately 1 micron thick (Figure 4A, B). The endothelium is continuous, overlying a collagenous lamina. Endothelial nuclei are located away from the surface and have irregular nuclei with rims of heterochromatin (Figure. 4A, B). Within the delicate stroma of the floor of the central cavity, regular paired ganglia travel along the length of the organ bilaterally. Progressively smaller nerves without neurons are present in the septa as they become finer. There are few small, random aggregates of lymphocytes that surround blood vessels and encroach on the epithelium with occasional areas of epithelial intercellular migration.
Figure 4:Atractosteus spatula; Transmission electron micrographs of
the gas bladder. A: The capillary barrier in the respiratory epithelium
consists of an outer microvillus border (thin black arrow) with a thin
cytoplasmic layer (thin white arrow), overlying a capillary (C) with a
continuous endothelial lining (black arrow) and interstitial layer in between
(asterisks).A red blood cell (RBC) is present in the lumen. The
thin white arrowhead indicates smooth muscle with filament bundles.
A nerve fiber (N), and a neuroendocrine cell (delimited by black arrowheads
and thick white arrow indicates nucleus) are present. B: The gas
bladder epithelium includes mucus cells (white arrowhead) and epithelial
cells with numerous microvilli (thin black arrow); mucus nuclei
(white arrow). L denotes the lumen. A Red Blood Cell (RBC) is in the
capillary. Multi lamellar bodies (white asterisk) are located in the apical
part of the respiratory epithelium. Smooth muscle cells (black arrowheads),
and striated muscle (black arrow) are present within a loose
collagenous stroma. C: The apical region of the epithelium includes
multi lamellar bodies (thick arrows), lumen (L), dense bodies (black arrowheads),
microvilli (thin black arrows), and a pneumocyte nucleus
(white arrowhead). D: (Overlapping field with A) The lamina propria
is composed of loose collagenous stroma populated by smooth muscle
(black arrow) and striated muscle (white arrow) with prominent mitochondria
(arrowhead). Nerves (N) are separated from the other components
by fibroblast extensions (asterisk).
Figure 5:Alligator gar; transmission electron microscope (TEM) micrographs
of the ABO. A: The white arrows indicate the pneumocyte nucleus
adjacent to the border of the epithelium. The double arrow heads
indicates the basal cytoplasmic extension just beneath them. Filament
bundle of smooth muscles (white arrowhead) show sub plasmalemmal
condensations (black arrow). B: This TEM micrograph shows the
neuroendocrine cell with dense bodies/ granules (white arrow) in its
cytoplasm, and the neuroendocrine is embraced with an extension of
fibroblast (black arrowhead). Lumen (L). C: Unmyelinated axons (arrowhead)
located between smooth muscles (black arrow) and striated
muscles (white arrow). The inset (TEM micrograph under higher magnification)
shows the unmyelinated axons that are embraced by the fibroblast
(asterisks).
Discussion
In air breathing fish, the general description of the gas
bladder, or ABO, is an unpaired oval elongated structure arising
from the posterior side of the pharynx and connected to the
pharynx. It is located dorsal to the body lumen and consists of a
central canal that occupies a third of the gas bladder and two lobes
that are covered with a very thin tissue wall [1,3,22,24,25,37].
Our findings in alligator gar were similar. The gas bladder was a
large elongate organ with progressively finer and shorter septa
forming first, second, third, and fourth order alveolar chambers
and a central canal. The gas bladders of other air breathing fish
are also described to have trabeculae dividing the gas bladder
into compartments and a central canal [1,30]. The alligator gar
gas bladder is medially divided into two alveolate portions by
a central canal lined by abundant ciliated epithelium, similar
to bowfins and other gar species [24,29,38]. The location and
abundance of ciliated cells suggests that these tissues function
as a pulmonary escalator and move cell and foreign debris to the
duct and then out into the pharynx.
Numerous mucus cells and surfactant bodies were identified in the respiratory epithelium, similar to that seen in L. oculatus [24]. The mucus content in mucus cells was an acid mucopolysaccharide, similar to that seen in non-respiratory airway surfaces in reptiles and mammals. Microvilli were abundant on the gas bladder surfaces of A. spatula, as was a secreted mucus barrier. We believe the functions of the microvilli, or ciliated cells, was to secrete mucus to protect the internal surface of the gas bladder as has been suggested in other gar [39].
Throughout the organ, smooth and skeletal muscle fascicles were abundant, giving strength to this delicate, well-vascularized organ. The muscle fascicles were often intimately associated with one another. The intermediate layer of the Lepisosteus gas bladder was described to consist of striated muscle, smooth muscle, fibroblasts, and elastic fibers [22]. We observed nerve bundles in the connective tissue of the alligator gar gas bladder. These findings were also reported in the spotted gar gas bladder [24]. Nerve fibers were also found in the walls of the ABO of bichir and gars [40, 41].
The nature of the opening to the gas bladder is debated in gars [24,29]. It has been anatomically described in multiple Lepisosteus species [1,24,29,38]. There is one brief description of a pneumatic duct in Atractosteus tristoechus [42]. Recent work in L. oculatus and L. osseus conclude that, in Lepisosteus, the alimentary canal opens into the gas bladder through a slitlike opening and that a discrete tubular structure, or “duct” is lacking [24,29]. In our study, we found that alligator gar have a pharyngeo-esophageal duct. It is anteriorly delimited by a slit-like opening and a slender aperture that continues as an epithelium lined lumen within a tubular structure circumscribed by connective tissue. This location is similar to the gas bladder opening described in L.oculatus, [24]. Unlike L. osseus, in which the gas bladder gradually widens from a narrow anterior point, the gas bladder in A. spatula begins where an abrupt termination of the glottal ridges of the pharyngeo-esophageal duct opens into a fully dilated, alveolated gas bladder with squamous respiratory epithelium. This epithelium is continuous with the central canal of the gas bladder. The glottal ridges of A. spatula are similar to those described in L. oculatus and L. osseus [24,29]. The presence of robust paired dorsal retractor muscles is also similar, and may control the size of the glottal opening.
In Lepisosteus, the interior surface of the respiratory epithelium is ciliated and lined with lamellar bodies and goblet cells [22]. In mammals, respiratory alveolar surfaces are lined by type I and type II pneumocytes. Type I cells are simple squamous cells, and type II cells are cuboidal [43-46]. Type II cells are associated with surfactant production and retain the capacity to divide, functioning as a source for new type I cells. Our study suggests that Atractosteus have one type of pneumocyte that probably combines the functions of mammalian type I and II pneumocytes. This pneumocyte was similar to the amphibian pneumocyte described by Pastor [46]. Amphibians, Dipnoi (lungfish), and L. oculatus also have one type of pneumocyte [46]. We observed some pneumocytes that appeared to have cytoplasmic processes. Similar cells were seen in the respiratory epithelium of amphibians [47-49]. Neuroepithelial cells occur between the pneumocytes and goblet cells in amphibians and Lepisosteus [37, 46]. In alligator gar, we observed that Neuroendocrine Cells (NECs) are located between epithelial cells and also between the mucus cells and ciliated cells in the respiratory epithelial cells as Neuroepithelial Bodies NEBs. Similar results were observed in spotted gar and long nose gar [24,38]. The presence of neuroendocrine cells suggests neuronal control of the gas bladder [37,38].
The internal surface of the alligator gar gas bladder is rich in ciliated cells that are irregular in shape and project toward the lumen of the gas bladder. These ciliated cells reduce the area available for respiratory exchange. Similar distribution of these ciliated cells has been observed in spotted gar and amphibians [24,48,50].
Alligator gar neuroendocrine cells were similar to those observed in the ABO of other gars. Neuroendocrine Cells (NECs) were pyramidal and occurred in the basal portion of the respiratory epithelium. These cells were characterized by the presence of dense secretory granules, Golgi bodies, rough endoplasmic reticulum, and scattered numbers of mitochondria in the basal part of the cell [46]. The presence of neuroendocrine cells in the gas bladder indicates endocrine and paracrine control over bladder function [37,38,46]. Neuroepithelial bodies and NEs are surrounded and invested by ciliated cells, goblet cells, and pneumocytes [37,38,46].
The alligator gar air breathing organ is a single air sac that emerges from the dorsal margin of the foregut, while amphibian lungs are paired organs that emerge from the ventral margin of the foregut [25,48]. There are many similarities in the anatomy and histology of the gar ABO and amphibian lungs [24].The alligator gar ABO has a series of air spaces similar to the lung but it lacks the bronchial tree that is observed in lungs [51,52]. The structure of the alligator ABO is very similar to the ABO of the genus Lepisosteus. Alligator gar inhabits coastal estuaries that are prone to agricultural and petrochemical pollution. One study reported that Atractosteus tropicus and Lepisosteus oculatus had higher tissue PCB concentrations than other fish in waters that had low, acceptable levels of contaminants [53]. Therefore, these fish are an excellent sentinel model for studying the effects of pollutants on aquatic ecosystems.
Numerous mucus cells and surfactant bodies were identified in the respiratory epithelium, similar to that seen in L. oculatus [24]. The mucus content in mucus cells was an acid mucopolysaccharide, similar to that seen in non-respiratory airway surfaces in reptiles and mammals. Microvilli were abundant on the gas bladder surfaces of A. spatula, as was a secreted mucus barrier. We believe the functions of the microvilli, or ciliated cells, was to secrete mucus to protect the internal surface of the gas bladder as has been suggested in other gar [39].
Throughout the organ, smooth and skeletal muscle fascicles were abundant, giving strength to this delicate, well-vascularized organ. The muscle fascicles were often intimately associated with one another. The intermediate layer of the Lepisosteus gas bladder was described to consist of striated muscle, smooth muscle, fibroblasts, and elastic fibers [22]. We observed nerve bundles in the connective tissue of the alligator gar gas bladder. These findings were also reported in the spotted gar gas bladder [24]. Nerve fibers were also found in the walls of the ABO of bichir and gars [40, 41].
The nature of the opening to the gas bladder is debated in gars [24,29]. It has been anatomically described in multiple Lepisosteus species [1,24,29,38]. There is one brief description of a pneumatic duct in Atractosteus tristoechus [42]. Recent work in L. oculatus and L. osseus conclude that, in Lepisosteus, the alimentary canal opens into the gas bladder through a slitlike opening and that a discrete tubular structure, or “duct” is lacking [24,29]. In our study, we found that alligator gar have a pharyngeo-esophageal duct. It is anteriorly delimited by a slit-like opening and a slender aperture that continues as an epithelium lined lumen within a tubular structure circumscribed by connective tissue. This location is similar to the gas bladder opening described in L.oculatus, [24]. Unlike L. osseus, in which the gas bladder gradually widens from a narrow anterior point, the gas bladder in A. spatula begins where an abrupt termination of the glottal ridges of the pharyngeo-esophageal duct opens into a fully dilated, alveolated gas bladder with squamous respiratory epithelium. This epithelium is continuous with the central canal of the gas bladder. The glottal ridges of A. spatula are similar to those described in L. oculatus and L. osseus [24,29]. The presence of robust paired dorsal retractor muscles is also similar, and may control the size of the glottal opening.
In Lepisosteus, the interior surface of the respiratory epithelium is ciliated and lined with lamellar bodies and goblet cells [22]. In mammals, respiratory alveolar surfaces are lined by type I and type II pneumocytes. Type I cells are simple squamous cells, and type II cells are cuboidal [43-46]. Type II cells are associated with surfactant production and retain the capacity to divide, functioning as a source for new type I cells. Our study suggests that Atractosteus have one type of pneumocyte that probably combines the functions of mammalian type I and II pneumocytes. This pneumocyte was similar to the amphibian pneumocyte described by Pastor [46]. Amphibians, Dipnoi (lungfish), and L. oculatus also have one type of pneumocyte [46]. We observed some pneumocytes that appeared to have cytoplasmic processes. Similar cells were seen in the respiratory epithelium of amphibians [47-49]. Neuroepithelial cells occur between the pneumocytes and goblet cells in amphibians and Lepisosteus [37, 46]. In alligator gar, we observed that Neuroendocrine Cells (NECs) are located between epithelial cells and also between the mucus cells and ciliated cells in the respiratory epithelial cells as Neuroepithelial Bodies NEBs. Similar results were observed in spotted gar and long nose gar [24,38]. The presence of neuroendocrine cells suggests neuronal control of the gas bladder [37,38].
The internal surface of the alligator gar gas bladder is rich in ciliated cells that are irregular in shape and project toward the lumen of the gas bladder. These ciliated cells reduce the area available for respiratory exchange. Similar distribution of these ciliated cells has been observed in spotted gar and amphibians [24,48,50].
Alligator gar neuroendocrine cells were similar to those observed in the ABO of other gars. Neuroendocrine Cells (NECs) were pyramidal and occurred in the basal portion of the respiratory epithelium. These cells were characterized by the presence of dense secretory granules, Golgi bodies, rough endoplasmic reticulum, and scattered numbers of mitochondria in the basal part of the cell [46]. The presence of neuroendocrine cells in the gas bladder indicates endocrine and paracrine control over bladder function [37,38,46]. Neuroepithelial bodies and NEs are surrounded and invested by ciliated cells, goblet cells, and pneumocytes [37,38,46].
The alligator gar air breathing organ is a single air sac that emerges from the dorsal margin of the foregut, while amphibian lungs are paired organs that emerge from the ventral margin of the foregut [25,48]. There are many similarities in the anatomy and histology of the gar ABO and amphibian lungs [24].The alligator gar ABO has a series of air spaces similar to the lung but it lacks the bronchial tree that is observed in lungs [51,52]. The structure of the alligator ABO is very similar to the ABO of the genus Lepisosteus. Alligator gar inhabits coastal estuaries that are prone to agricultural and petrochemical pollution. One study reported that Atractosteus tropicus and Lepisosteus oculatus had higher tissue PCB concentrations than other fish in waters that had low, acceptable levels of contaminants [53]. Therefore, these fish are an excellent sentinel model for studying the effects of pollutants on aquatic ecosystems.
Table 3: Demographics data and the time to blockade (m ± SD).
ReferencesTop